Metastasis, the process in which cancer cells spread from the original tumor to distant organs, is responsible for nearly 90 % of cancer-related deaths. This process begins when cancer cells invade surrounding tissue, enter either the lymphatic vessels or blood vessels, and travel through the circulation to other parts of the body, where they form new tumors. While therapies for primary tumors have become more effective over the past decades, treating metastatic tumors continues to be a major clinical challenge. One promising strategy is the use of chemotherapeutic agents that can trigger a process called immunogenic cell death (ICD). Unlike ordinary forms of cell death, ICD stimulates the immune system to recognize and attack cancer cells. This is important because it can reduce the risk of tumor recurrence and prevent the spread of tumors. Our research group is studying gallium(III) complexes as potential agents capable of inducing immunogenic cell death (Figure 1).
Figure 1. Structure of Ga(III) complex as an ICD inducing agent.
A characteristic feature of ICD is the movement of calreticulin, a protein usually found inside the cell, to the surface of the cell. When calreticulin appears on the surface, it functions as an “eat me” signal that attracts immune cells such as macrophages and dendritic cells. These immune cells then start the process of presenting tumor antigens to the immune system. Using flow cytometry, we tested whether the complex could cause this effect. The treatment with the complex triggered the calreticulin exposure on the surface of the cancer cells in a dose-dependent manner (Figure 2A). Interestingly, the percentage of calreticulin-positive cells was higher after treatment with our complex than after treatment with oxaliplatin, a well-known inducer of ICD. The phosphorylation of the protein eukaryotic initiation factor 2 alpha, a key step in the endoplasmic reticulum stress response, was observed. In agreement with the findings on calreticulin, treatment with the complex resulted in an increased level of phosphorylated eukaryotic initiation factor 2 alpha in cancer cells (Figure 2B). Other molecules associated with ICD, known as damage-associated molecular patterns, were also released after treatment. Increased extracellular levels of adenosine triphosphate (Figure 2C) and the protein high mobility group box 1 (Figure 2D) were detected. Both molecules function as signals that alert and activate the immune system.
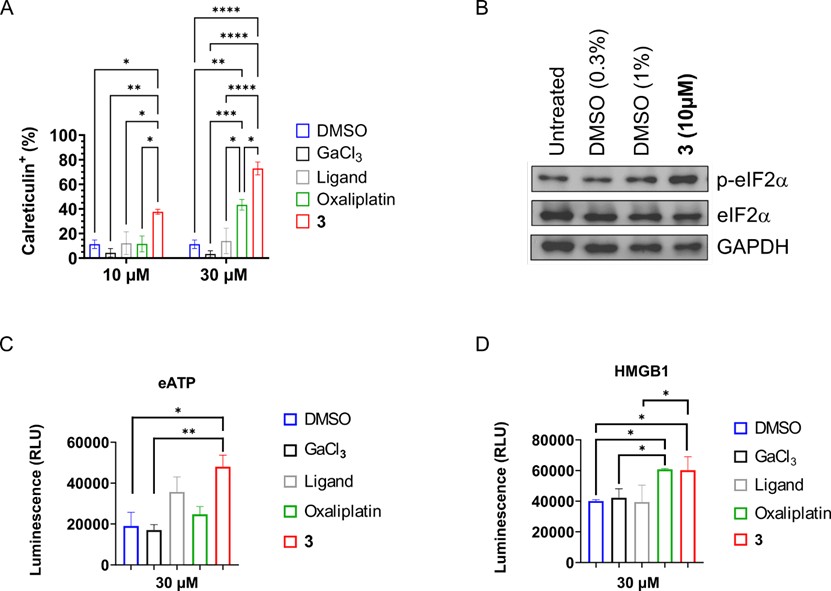
Figure 2. Hallmarks of ICD induced by the Ga(III) complex (3). A) Percentage of cancer cells showing calreticulin on the surface. B) Western Blot analysis of (phosphorylated) eukaryotic initiation factor 2 alpha refrenced to glyceraldehyde 3-phosphate dehydrogenase.C) Release of extracellular adenosine triphosphate. D) Release of high mobility group box 1 protein.
Since dendritic cells play a central role in the activation of anti-tumor T cells, the ability of the released signals to stimulate dendritic cells was examined. Peripheral blood mononuclear cells, which contain dendritic cells, were co-cultured with cancer cells pre-treated with the gallium(III) complex. Under these conditions, dendritic cells displayed increased levels of the activation markers cluster of differentiation 80 and cluster of differentiation 86 (Figure 3A-C). Taken together, these observations indicate that the gallium(III) complex not only destroys cancer cells but also stimulates the immune system to recognize and respond to them. This dual mode of action suggests potential usefulness in eliminating primary tumors as well as in preventing metastasis and tumor recurrence.
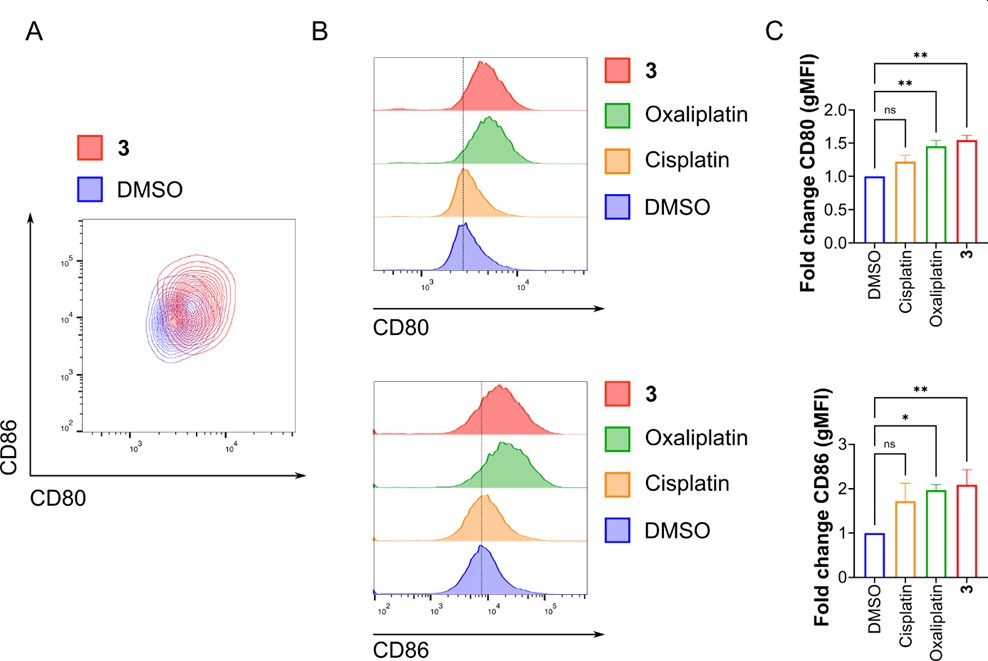
Figure 3. Activation of dendritic cells after exposure to cancer cells treated with Ga(III) complex (3). A) Contour plot showing expression of CD80 and CD86 in dendritic cells co-cultured with cancer cells pre-treated with the complex. B) Histograms showing expression levels of CD80 and CD86. C) Bar graphs represent fold change in geometric mean fluorescence intensity of CD80 and CD86.
See our recent TV interview (in German) at the WDR, starting at 14:50 min
Key References
[1] L. Zhang, N. Montesdeoca, J. Karges*, H. Xiao*, Immunogenic Cell Death Inducing Metal Complexes for Cancer Therapy, Angew. Chem. Int. Ed. 2023, 62, e202300662. DOI: http://dx.doi.org/10.1002/anie.202300662
[2] Z. Papadopoulos, Y. Antar, I.-S. Dieter, F. Peeters, C. Plaza-Sirvent*, J. Karges*, Gallium(III) Complex Induces Immunogenic Cell Death Hallmarks for Chemoimmunotherapy, J. Med. Chem. 2025, 68, 15980-15990. DOI: http://dx.doi.org/10.1021/acs.jmedchem.5c00969
[3] K. Shang, N. Montesdeoca, H. Zhang, E. Efanova, G. Liang, J. Ochs, J. Karges*, H. Song,* L. Zhang*, Cobalt(III) Prodrug-Based Nanomedicine for Inducing Immunogenic Cell Death and Enhancing Chemo-Immunotherapy, J. Control. Release 2024, 373, 493-506. DOI: http://dx.doi.org/10.1016/j.jconrel.2024.07.042
[4] H. Zhou, D. Tang, Y. Yu, L. Zhang, B. Wang, J. Karges*, H. Xiao*, Theranostic Imaging and Multimodal Photodynamic Therapy and Immunotherapy using the mTOR Signaling Pathway, Nature Commun. 2023, 14, 5350. DOI: http://dx.doi.org/10.1038/s41467-023-40826-5